Sodium Ethoxide: Exploring Reactions and Applications
|
is a crucial organic compound with extensive applications in the chemical industry. It plays a significant role in organic synthesis, catalyst preparation, and various other chemical reactions. This article will delve into the chemical reaction mechanisms, physical properties, and application cases of sodium ethoxide to provide a comprehensive understanding of this important compound. Physical Properties
Sodium ethoxide appears as a white or pale yellow crystalline powder, easily soluble in ethanol and ether. However, it is hygroscopic and decomposes when exposed to air. Therefore, it should be stored in a sealed, light-proof container to prevent moisture absorption and degradation. Chemical Reaction Mechanism
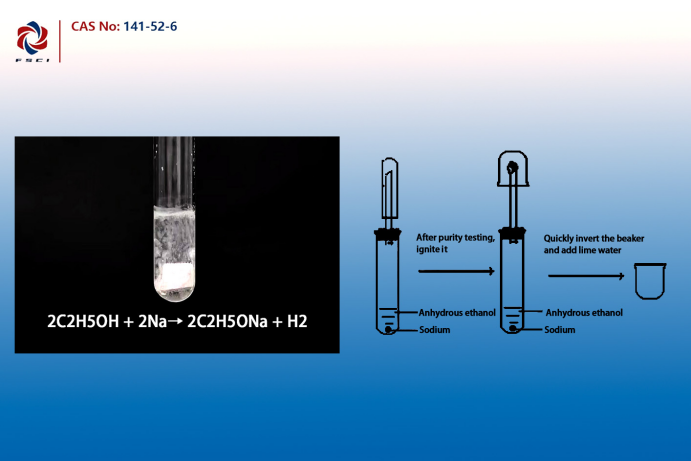
Sodium ethoxide is typically prepared by reacting ethanol with metallic sodium.In organic synthesis, sodium ethoxide exhibits strong basicity and nucleophilicity, commonly used in E2 and S_N2 reactions, promoting the dehydrohalogenation and etherification processes. Its dissociation in solution makes it an effective nucleophilic reagent, participating in various organic reactions. Applications in Organic SynthesisIn organic synthesis, sodium ethoxide is widely used as a strong base and nucleophilic reagent to prepare ethers, esters, and ketones. For example, in pharmaceutical synthesis, sodium ethoxide is used to catalyze hydroxylation and cyclization reactions, ensuring efficient reactions and high-purity products.
Catalyst PreparationSodium ethoxide also plays an essential role in preparing organometallic catalysts. By reacting with metal compounds to form metal ethoxides, these catalysts exhibit excellent activity and selectivity in both homogeneous and heterogeneous catalytic reactions.
Industrial ApplicationsIn industrial production, sodium ethoxide is used to manufacture dyes, pesticides, and other chemical intermediates. For example, in the dye industry, sodium ethoxide catalyzes the synthesis of aniline compounds, significantly improving production efficiency.
New Technologies and InnovationsWith the promotion of green chemistry and sustainable development concepts, the application of sodium ethoxide in environmentally friendly reactions is becoming increasingly widespread. Emerging technologies such as microfluidic reactors and solvent-free synthesis not only enhance the utilization efficiency of sodium ethoxide but also reduce the environmental impact of chemical reactions.
ConclusionAs a versatile organic compound, sodium ethoxide has broad application prospects in the chemical industry. By deeply understanding its chemical reaction mechanisms, physical properties, and application cases, can better utilize this compound to advance various chemical reactions and industrial production processes. Our company is committed to providing customers with high-purity, high-quality sodium ethoxide products and comprehensive technical support. For more information and solutions, please contact us. Contact us
|
 |


 EN
EN
 AR
AR
 BG
BG
 HR
HR
 CS
CS
 DA
DA
 NL
NL
 FI
FI
 FR
FR
 DE
DE
 EL
EL
 HI
HI
 IT
IT
 JA
JA
 KO
KO
 NO
NO
 PL
PL
 PT
PT
 RO
RO
 RU
RU
 ES
ES
 SV
SV
 TL
TL
 IW
IW
 ID
ID
 LV
LV
 LT
LT
 SR
SR
 SK
SK
 VI
VI
 HU
HU
 TH
TH
 TR
TR
 GA
GA
 CY
CY
 KA
KA
 LA
LA
 MN
MN
 KK
KK
 LB
LB